Myeloma is a blood cancer that affects a particular type of white cells called plasma cells. There are approximately 1,800 new cases of myeloma diagnosed in Australia each year.
Myeloma patients could present with different symptoms; these include bone pain from bony lesions/fractures, breathlessness and fatigue due to low red cell count (anaemia), kidney damage and recurrent infections. If you have concerns regarding any of these symptoms – please discuss with your GP about potentially getting further investigations for myeloma.
Currently, myeloma is not curable. The aim of treatment for myeloma patients is to produce durable control of the disease. In general, myeloma treatment is divided into 3 phases – induction, consolidation and maintenance – depending on patients’ age and fitness level.
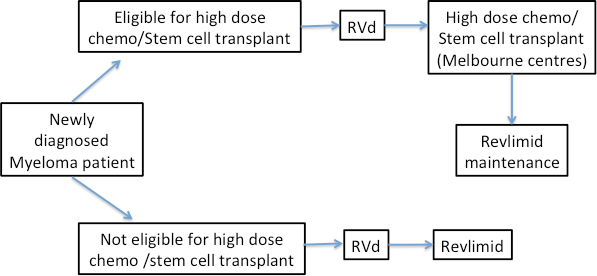
Following initial treatment (induction), patients age < 70 years old, fit and with no significant health issues will be considered for consolidation treatment of high dose chemotherapy and stem cell transplant in one of the tertiary hospitals in Melbourne.
For many years, myeloma patients in Australia have received either Velcade (Bortezomib) injection under the skin (subcutaneous), or Revlimid (Lenalidomide) oral tablet, to start their treatment when first diagnosed with myeloma. Velcade works by causing accumulation of ‘waste products’ within the myeloma cells causing them to self-destruct. Revlimid, on the other hand, is effective in eliminating myeloma cells through multiple mechanisms including modulating the body’s immune system.
Starting from 1st June 2020, Pharmaceutical Benefits Scheme (PBS) has approved the treatment of myeloma patients in Australia with “triplet therapy” – using combination of Revlimid/Velcade/Dexamethasone (RVd) treatment as first line therapy, regardless of whether patients will be eligible for consolidation treatment with high dose chemotherapy and stem cell transplantation later on.
Figure 1 – Treatment journey for myeloma patients in Australia with new RVd “triplet therapy” approved in June 2020
Clinical trials have shown that the triplet combination improves survival in myeloma patients, compared to if the drugs are used individually. Maximizing treatment for myeloma patients upfront provides the best opportunity to optimize long-term outcome. Our haematologists at Ballarat Cancer Care and Haematology would be available for further discussion about this new treatment.
Dr Pohan Lukito